The Food and Drug Administration is solidifying its standards to neutralize what some have called a Wild West of immune response testing for the coronavirus.
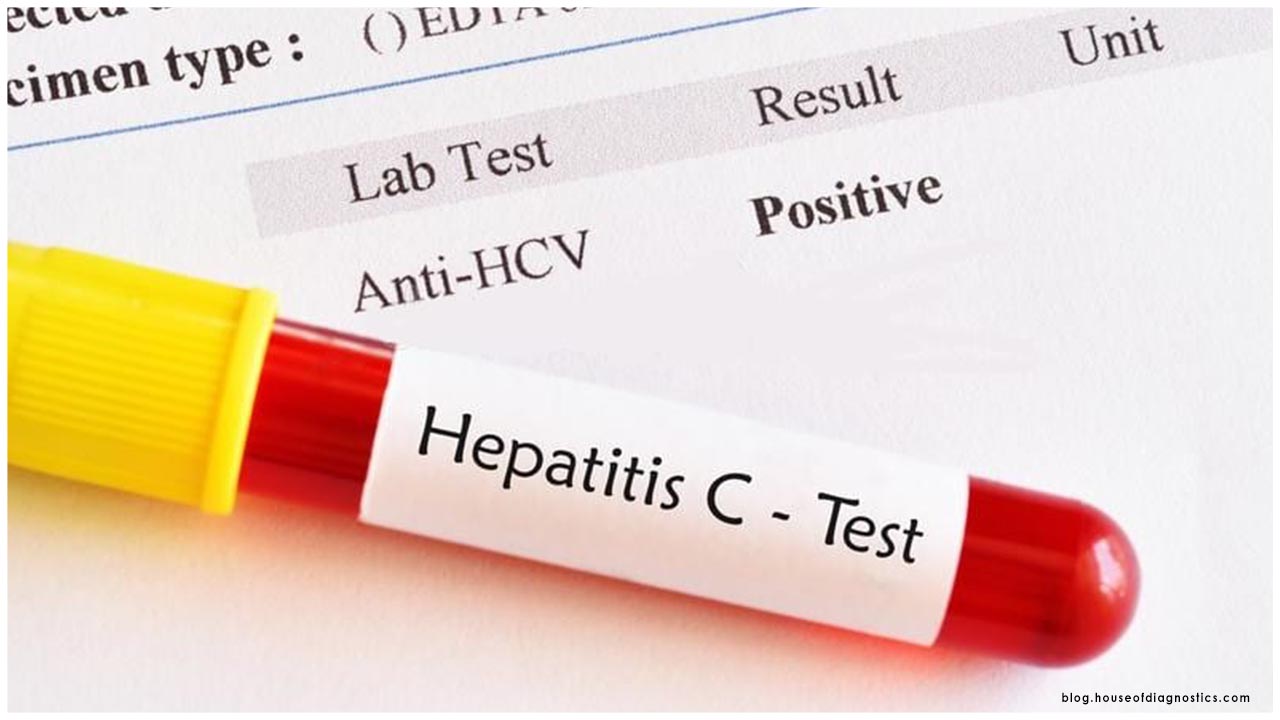
These tests are intended to distinguish individuals who have been recently presented to the infection. The FDA said more than 250 designers have been acquiring items to the market the previous scarcely any weeks.
In a race to make immunizer tests accessible as fast as could be expected under the circumstances, the FDA had set a low standard for these tests. Makers should present their data about the precision of their products, however, the organization had no guidelines for what might be satisfactory. Organizations weren't permitted to guarantee the tests were approved by the FDA, under the starting direction gave in mid-March.
Presently the FDA is telling makers that on the off chance that they need their tests to stay available, they should fulfill the least quality guidelines and present a solicitation for crisis use approval, a brief course to showcase for unapproved items when others aren't accessible. The EUA includes a lower standard than the typical FDA leeway or endorsement.
The FDA said 12 makers have just picked to demand EUA's for their items. More than 100 different makers have been conversing with the office about utilizing this procedure, said FDA Commissioner Stephen Hahn. He talked on a press call Monday. Organizations have 10 days to present that demand.
"We desire that the individuals who can't [meet the new standard] will pull back their items from the market and we will be working with them to assist them with doing that," he said.
These tests are currently so far-reaching that individuals can arrange them from lab mammoths Quest or LabCorp. The tests can cost more than $100. Even though the FDA's unique direction requires these tests to be controlled by an affirmed lab, the packs themselves are easy to utilize and have been promptly accessible.
Despite the eagerness encompassing these tests, they have significant confinements. Even though individuals who test constructive for antibodies have much of the time been presented to the coronavirus, researchers don't know whether that implies those individuals are invulnerable from the coronavirus, and if so for to what extent.
"Regardless of whether this is the ticket for somebody to return to work [based exclusively on an immune response test result], my conclusion on that would be no," Hahn said.
The tests might be progressively valuable when joined with data from a standard coronavirus analytic test, or is somebody who has indications, or if the outcomes have been affirmed with an alternate immune response test. That "would significantly expand the exactness of those tests," said Jeffrey Shuren, executive of the FDA's Center for Devices and Radiological Health
Antibodies are a conceivably important research apparatus and can be utilized to decide the pervasiveness of a malady in a populace. In that situation, individual bogus outcomes are less significant. New York State utilized counteracting agent tests to establish that around 20 percent of individuals in New York City has just been presented to the coronavirus.
In California, specialists have endeavored to quantify the commonness of the coronavirus in Los Angeles County and Santa Clara County in the Bay Area. Those unpublished outcomes have accumulated analysis because even a test that is more than 99 percent precise can deliver numerous bogus constructive outcomes when used to study hundreds or thousands of individuals.
Even with this analysis, the creators of the Santa Clara study have posted reexamined results recognizing the high level of vulnerability in their discoveries. Those discoveries haven't been peer-assessed.
The crisis use approval is just substantial during the hour of the national crisis. "When the national crisis closes, the EUA approvals end also," Shuren said. Organizations that need to continue promoting these tests should get them endorsed through the customary, progressively rigid FDA process.
FDA authorities state they will keep on taking action against organizations that erroneously guarantee their tests are endorsed by the FDA, or that advertise them for home use, which isn't presently permitted.

 In the latest progress on Testing FDA helps a antibody test for covid 19
In the latest progress on Testing FDA helps a antibody test for covid 19