The U.S. Food and Drug Administration has given the principal crisis use approval (EUA) for a COVID-19 antigen test, another class of tests for use in the continuous pandemic. These indicative tests rapidly identify pieces of proteins found on or inside the infection by testing tests gathered from the nasal pit utilizing swabs. The EUA was given late Friday to Quidel Corporation for the Sofia 2 SARS Antigen FIA. This test is approved for use in high and moderate multifaceted nature research centers guaranteed by Clinical Laboratory Improvement Amendments (CLIA), just as for purpose of-care testing by offices working under a CLIA Certificate of Waiver.
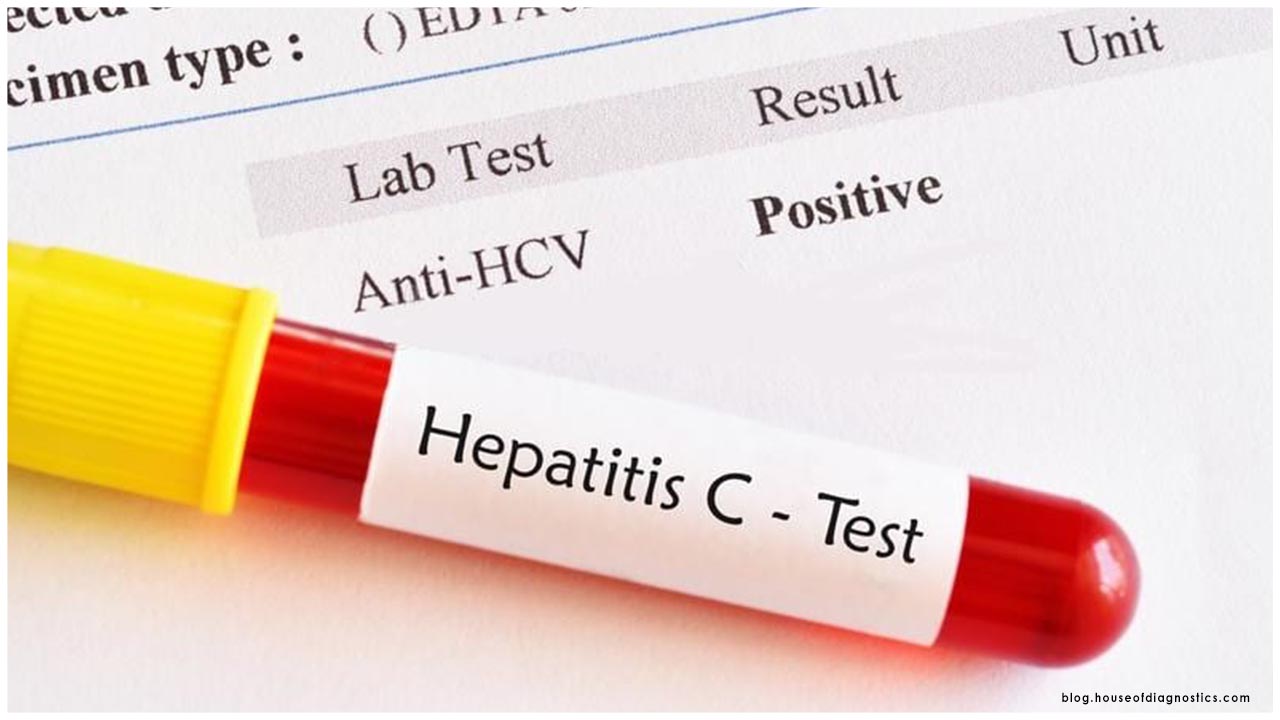
Symptomatic testing is one of the mainstays of our country's reaction to COVID-19 and the FDA keeps on taking activities to help make these basic items accessible, including by giving EUAs. During this pandemic, there have been two sorts of tests for which the FDA has given EUAs. One sort is polymerase chain response (PCR) tests, a sub-atomic analytic testing strategy that identifies the hereditary material from the infection and can help analyze functioning COVID-19 contamination. The other sort is serological tests that search for antibodies to the infection, which can help recognize people who have built up a versatile resistant reaction to the infection, as a feature of either functioning contamination or an earlier disease (serological, or immune response, tests ought not to be utilized to analyze dynamic contamination).
This most recent FDA approval is for an antigen test, which is another kind of indicative test intended for fast identification of the infection that causes COVID-19. Every classification of the analytic test has its one of a kind job in the battle against this infection. PCR tests can be unfathomably precise, however, running the tests and breaking down the outcomes can require some serious energy. One of the fundamental points of interest of an antigen test is the speed of the test, which can give brings about minutes. Be that as it may, antigen tests may not identify every single dynamic contamination, as they don't work a similar route as a PCR test. Antigen tests are quite certain for the infection, yet are not as touchy as atomic PCR tests. This implies positive outcomes from antigen tests are exceptionally precise, yet there is a higher possibility of bogus negatives, so negative outcomes don't preclude contamination. In light of this, negative outcomes from an antigen test may be affirmed with a PCR test before settling on treatment choices or to forestall the conceivable spread of the infection because of a bogus negative.
Antigen tests are additionally significant in the general reaction against COVID-19 as they can, for the most part, be delivered at a lower cost than PCR tests and once different producers enter the market, can conceivably scale to test a large number of Americans every day because of their less complex structure, helping our nation better distinguish disease rates nearer to constant.
This is only the principal antigen test to be approved and we hope for something else to follow. We likewise envision giving a EUA format to antigen tests, like ones we've discharged for other test types, to assist makers with smoothing out entries and help speed up our survey and issuance of extra EUAs.
Antigen tests will assume a basic job in the battle against COVID-19 and we will keep on offering backing and aptitude to help with the advancement of precise tests, and to audit and screen advertised tests to guarantee the exactness, while adjusting the pressing requirement for these basic diagnostics.
The FDA, an office inside the U.S. Division of Health and Human Services, ensures the general wellbeing by guaranteeing the wellbeing, viability, and security of human and veterinary medications, immunizations, and other natural items for human use, and clinical gadgets. The organization likewise is liable for the wellbeing and security of our country's food flexibly, makeup, dietary enhancements, items that emit electronic radiation, and for controlling tobacco items.

 Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients Statement from Director - CDRH Offices: Office of the Center Director
Dr. Jeffrey E. Shuren MD, JD
Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients Statement from Director - CDRH Offices: Office of the Center Director
Dr. Jeffrey E. Shuren MD, JD